The pharmaceutical industry demands the highest levels of water purity for manufacturing drugs, injectables, and sterile products. In this environment, the Purified Water Storage and Distribution System plays a critical role in maintaining water quality and ensuring compliance with regulatory requirements such as those set by the USP, EP, and WHO.
Pharmaceutical water systems must be designed to eliminate the risk of microbial contamination and endotoxins. A typical system consists of a high-purity storage tank, distribution loop, and sanitary fittings, all constructed with SS316L stainless steel. The loop is maintained under continuous recirculation to avoid stagnation, typically at a minimum velocity of 1.5 meters per second. Dead legs are minimized or completely avoided by precise engineering and orbital welding, which ensures smooth and crevice-free joints.
The distribution system is automated using PLC or SCADA controls, offering real-time monitoring of key parameters like flow, temperature, conductivity, and Total Organic Carbon (TOC). Clean-in-Place (CIP) and periodic hot water or ozone sanitization are incorporated to maintain microbial control. Validated systems also generate audit trails and reports required for GMP and FDA compliance.
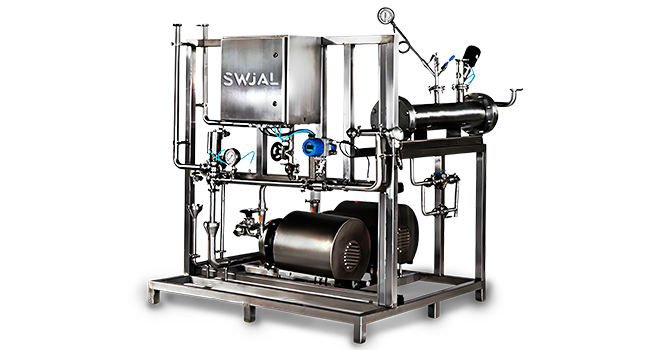 |
| Pharmaceutical High Purity Water Storage and Distribution Plant |
Applications in this sector include water for oral liquids, injections, cleaning of equipment, and rinsing of containers. Any compromise in water quality could result in product recalls, making these systems not just a utility, but a cornerstone of product safety.
In addition to quality and compliance, operational efficiency is another major advantage of using advanced purified water storage and distribution systems. With integrated automation, pharmaceutical manufacturers can track system performance in real-time, predict maintenance needs, and reduce downtime. This results in lower operational costs and higher productivity. The flexibility of design also allows the system to scale as production needs increase, making it future-proof and adaptable to expanding business requirements.
Moreover, energy efficiency and sustainability have become critical factors in pharmaceutical manufacturing. Modern water systems incorporate energy-saving features such as variable frequency drives (VFDs), heat recovery units, and eco-friendly sanitization methods. By investing in such sustainable systems, pharmaceutical companies not only reduce their environmental footprint but also demonstrate commitment to responsible manufacturing practices.
In conclusion, a well-designed and validated Purified Water Storage and Distribution System ensures consistent quality, operational efficiency, and regulatory compliance in pharmaceutical manufacturing.
No comments:
Post a Comment